srne stock news fda approval
Published Mar 2 2021 518PM EST RTTNews - Shares of Sorrento Therapeutics Inc. SRNE Sorrento has received a supplemental new.

Sorrento Publishes An Abivertinib Teaser Entitled Abivertinib A Franchise Oral Therapeutic For Cancer Covid 19 And Autoimmune Diseases
SRNE Sorrento and its majority-owned subsidiary Scilex Pharmaceuticals Inc.

. 2022-03-02 1124 ET - News Release. Biotech Stock Catalyst and FDA Calendar for your biotech stock investing. Dulan Lokuwithana SA News Editor Grandbrothers.
SRNE Sorrento previously announced that its partner Escugen Biotechnology Co Ltd. RECEIVES APPROVAL FOR MASTERPLAN FOR ALGODON WINE ESTATES 4138 ACRE LUXURY WINE WELLNESS DEVELOPMENT IN MENDOZA ARGENTINA VINO Feb 9 2022 1019 AM. 22 rows SAN DIEGO Jan.
Ad 1000 Strong Buy Stocks that Double the SP. The company said that the conjugate takes advantage of several technology platforms under development. Biotech stocks with key binary eventscatalysts - FDA ApprovalPDUFA dates Advisory Committee and Phase 2 3 trial data releases dates are noted.
SRNE Stock Heads Up On FDA IND Approval In the press release Sorrento Therapeutics said that it received clearance from the FDA for its IND for STI-6129. Phase 1 catalysts for small-cap companies only are listed. Sorrento is planning to meet with FDA and EMA to discuss the data package and gain concurrence on the path forward to a BLA or MAA.
Sorrento Therapeutics - We apply cutting-edge science to create innovative therapies that will improve the lives of those who suffer from cancer intractable pain and COVID-19. Levena had received an approval letter from the Center for Drug Evaluation CDE of the National Medical Products Administration NMPA for its Application for Clinical. Actual approval is not typically transmitted only questions or denial.
FDA Approval Highlights High-Risk Case for Sorrento Therapeutics Stock With multiple ways to win SRNE stock looks attractive here By InvestorPlace Research Staff Sep 23 2020 755 am EST. Thus we should be expecting a new trial protocol on the FDA clinical. March 2 2022.
Scilex Holding Scilex an over 99 owned subsidiary of Sorrento Therapeutics NASDAQ. 02 2022 1202 PM ET Sorrento Therapeutics Inc. FDA granted IND clearance today for STI-9199 COVISHIELD TM for a Phase 1 safety and pharmacokinetic study in healthy volunteers.
We are pleased to announce that 121 Mining Investment Las Vegas taking place at The Venetian from the 30-31 March is sold out to miners. Sorrento rises 7 as STI-1558 neutralizes. SRNE Sorrento has received a supplemental new drug application sNDA approval from the FDA for ZTlido to make efficacy labeling change with clinical data.
Sorrento granted FDA nod to start clinical trial for intranasal COVID-19 therapy Mar. STI-6129 is a CD38-targeting antibody drug conjugate. Because if SRNE receives approval than they will act as a symbol of hope in the meantime.
Scilex Holding Scilex an over 99 owned subsidiary of Sorrento Therapeutics NASDAQ. Scilex received approval from the US. Sorrento Therapeutics Inc.
ZTlido was approved by the FDA on February 28 2018. Sorrento Announces FDA Authorization to Proceed With Phase 1 Study Of Intranasal STI-9199 COVISHIELD a Potent Neutralization Antibody Against Covid-19 Viruses. SRNE stock news and headlines to help you in your trading and investing decisions.
Report shares skyrocketed Thursday after the biopharmaceutical company received Food and Drug Administration approval for a Phase 1. Food and Drug Administration. SRNE gained over 5 in extended trading session on Tuesday after the company FDA cleared its Investigational New.
We use a technical analy. 28 2022 GLOBE NEWSWIRE -- Sorrento Therapeutics Inc. 1 hour agoStock Market News.
Sorrento Therapeutics stock get FDA clearance for Phase 2 Abivertinib trial study T-VIVA. Use our tools on your road to profit in the stock market. COVISHIELD IND and Clinical Trial.
Sorrento Therapeutics SRNE - Get Sorrento Therapeutics Inc. SRNE Business Wire - 1312022 51300 PM. Should you buy Sorrento stock now SRNE.
The Chinese approval covers 6 different indications including adult ulcerative colitis ankylosing spondylitis rheumatoid arthritis adult and childhood Crohns disease fistula Crohns disease and psoriasis. TSK Feb 9 2022 900 AM. Amended Statement of Ownership sc 13ga Edgar US Regulatory - 272022 44216 PM Current Report Filing 8-k Edgar US Regulatory - 212022 40535 PM Newman Ferrara LLP Announces Corporate Governance Investigation of Sorrento Therapeutics Inc.
Escugen and Sorrentos subsidiary Levena Suzhou Biopharma Co Ltd. Get the latest Sorrento Therapeutics Inc. 55 minutes agoSorrento Therapeutics Inc.
Since the recent PR stated that the IND filing was submitted accepted February 1st there is a 30 day window for approval which is in a couple of days. SRNE received clearance from the FDA for its investigational new drug application IND for intranasal IN STI-9199 COVISHIELD to study the safety and.

This Analyst Sees A 14 Bagger In Sorrento Srne Stock Nasdaq

Will Sorrento Therapeutics Stock Continue To Make Fresh Lows Nasdaq
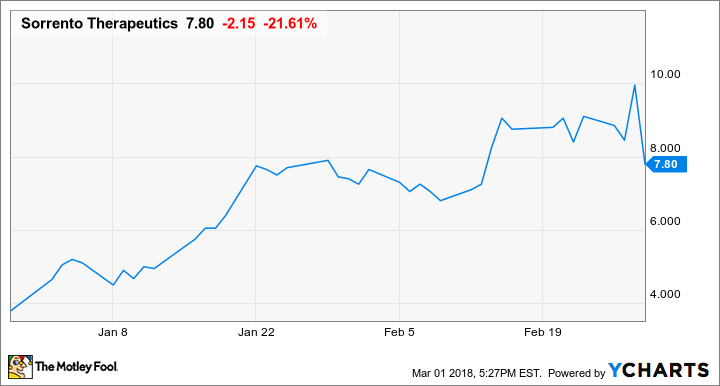
Why An Fda Approval Caused Sorrento Therapeutics Pain Markets Stocks Billingsgazette Com

Where Will Sorrento Therapeutics Be In 1 Year The Motley Fool
Sorrento Stock Price Soars 27 As Covid 19 Antibody Trial Gets Green Light

Sorrento Therapeutics Inc Srne Stock Forum Discussion Yahoo Finance
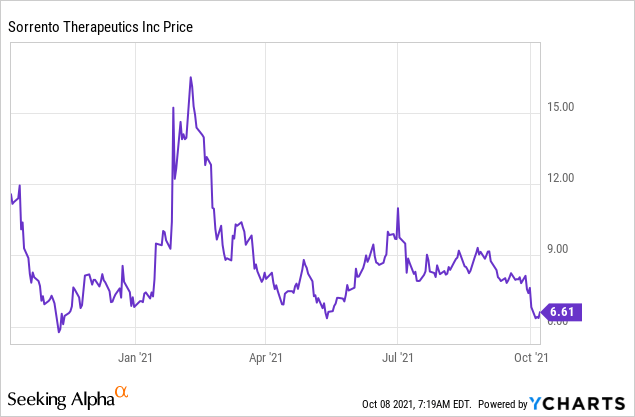
Sorrento Therapeutics Wide Oncology Pipeline And Watch Out For Covid Updates Seeking Alpha

Is Sorrento Therapeutics A Ticking Time Bomb For Investors The Motley Fool

Sorrento Stock Is A Buy Because Of Its Strong Drug Pipeline Says Analyst Nasdaq
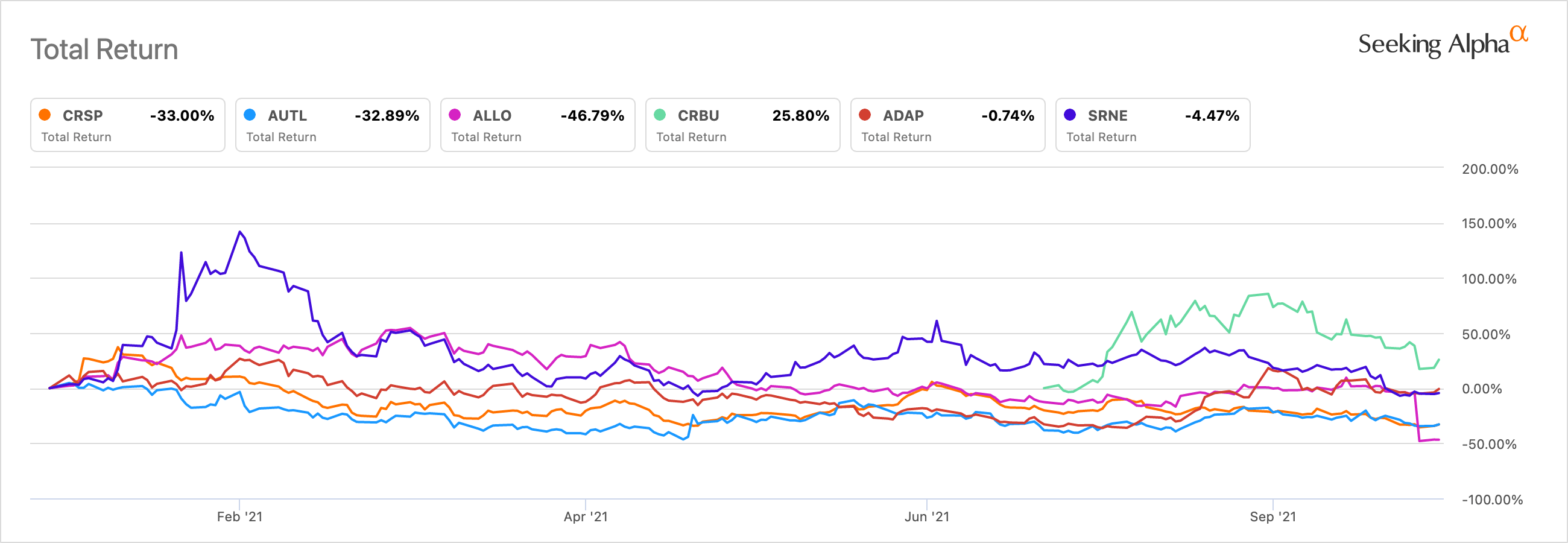
As Crispr Continues To Falter Other Car T Names Aren T Impacted Seeking Alpha

Is Sorrento Therapeutics Stock A Buy The Motley Fool

Will Sorrento Therapeutics Stock Continue To Make Fresh Lows
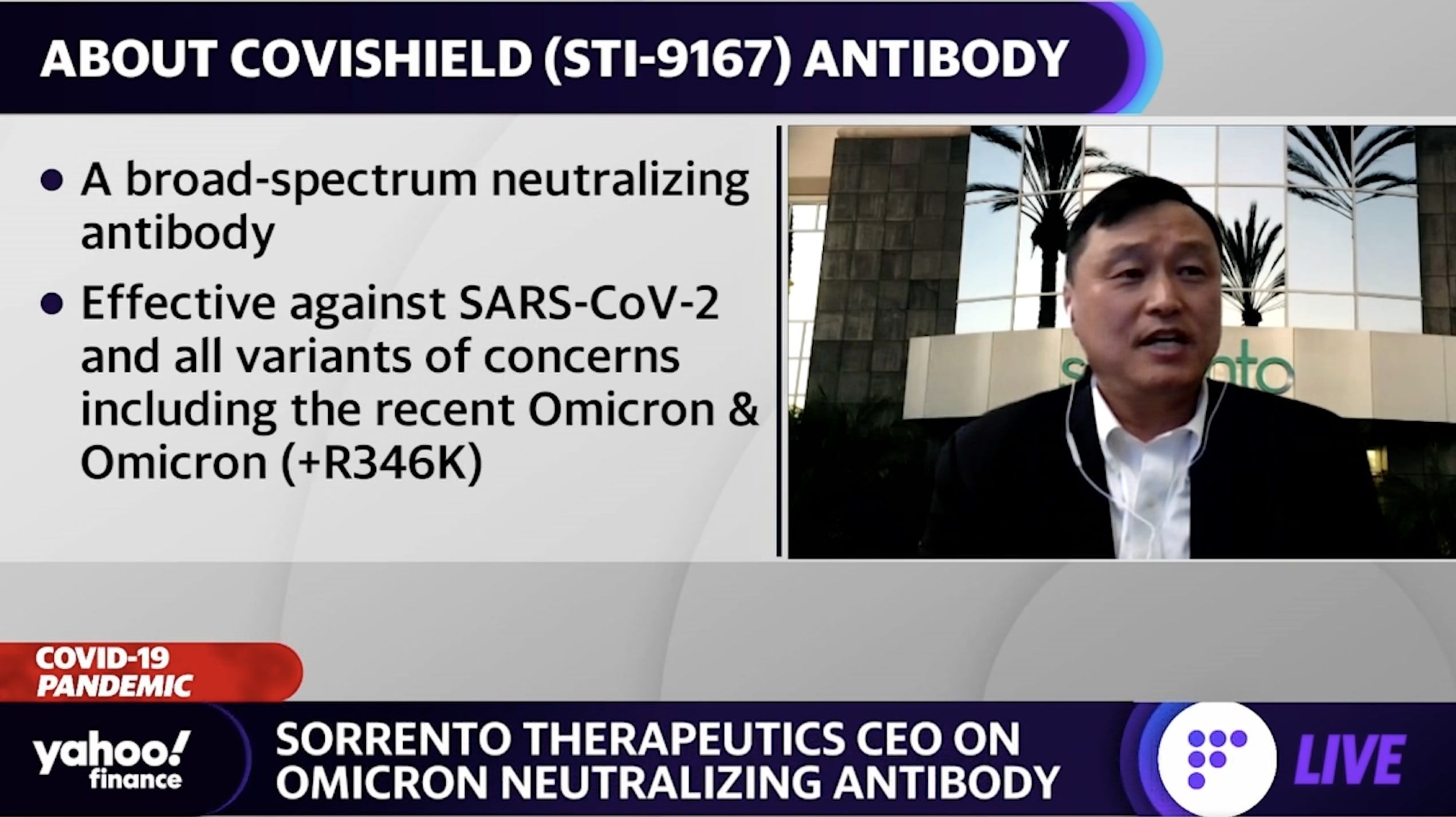
Sorrento Therapeutics Ceo Details Covishield Neutralizing Antibody

Pipeline Sorrento Therapeutics

First Week Of January 2024 Options Trading For Sorrento Therapeutics Srne Nasdaq